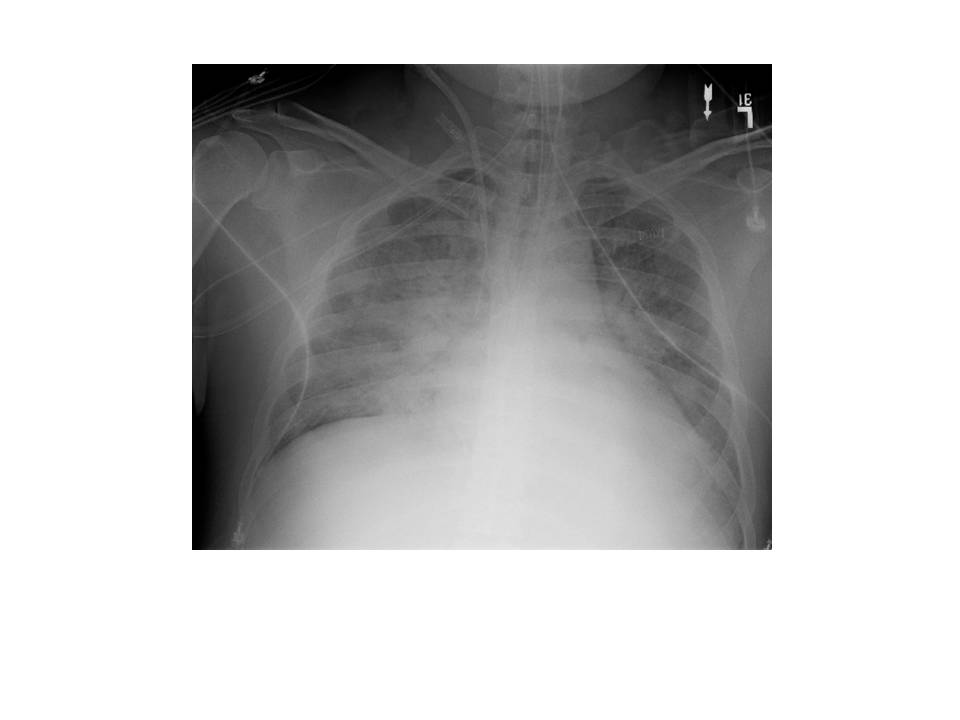
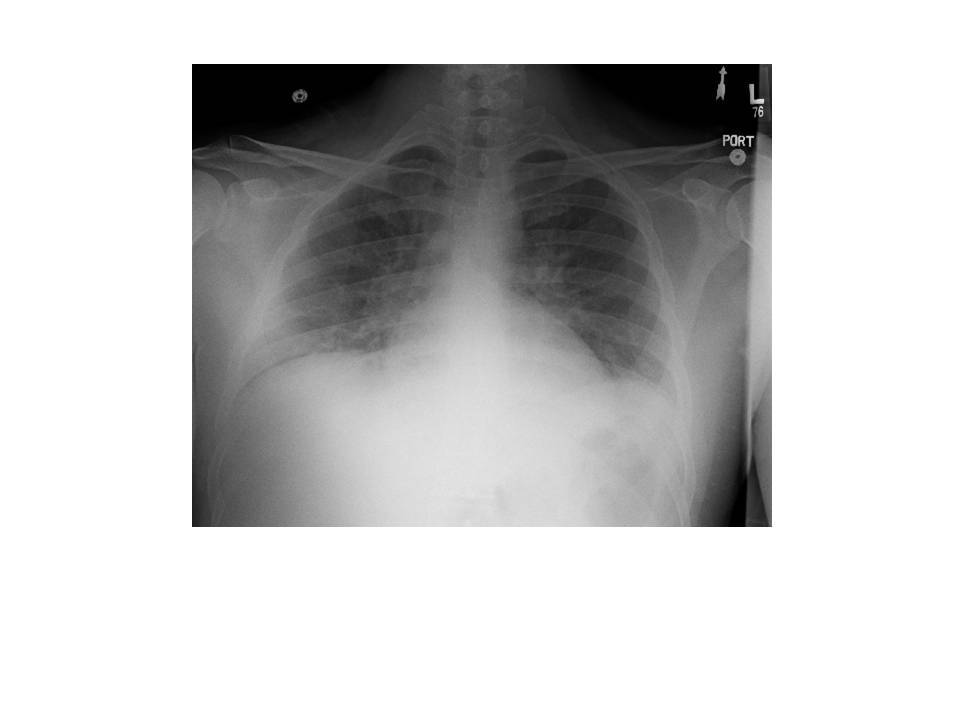
Cardiac work-up was notable for an elevated troponin and an echocardiogram showing global wall motion abnormalities, worse in the inferolateral, inferior and inferoseptal wall segments with LV systolic dysfunction and an estimated ejection fraction of approximately 25%.
Rationale: As with the initial management of any patient of septic shock, this patient should receive intravenous antibiotic therapy as early as possible (within the first hour) after diagnosis (5). Antibiotics should be directed at the most likely source, an intra-abdominal infection.
The Surgical Infection Society’s published guidelines regarding antimicrobial therapy for intra-abdominal infections (6), recommend treating with agents effective against aerobic, facultative anaerobic Enterobactericae and anaerobic organisms, particularly Bacteroides fragilis (see Table 1). Although both single agents and combination regimens have been identified to be effective in the control of intra-abdominal infections in case series and clinical trials, these trials have been designed to demonstrate therapeutic equivalence and have not been powered to demonstrate major effectiveness among them. Moreover, there have been few prospective, multivariate analyses aimed at identifying risk factors for treatment failure and death in patients with intra-abdominal infections. Most of these factors relate to the patient’s underlying physiological status and response to the infection. Physiologic risk factors for poor outcome include advanced age, poor nutritional status, low serum albumin concentration, preexisting medical disorders (especially significant cardiovascular disease) and the most consistently recognized risk factor being a higher APACHE II score. This high risk factor group of patients should be treated with broader spectrum antimicrobial regimen, covering gram-negative, aerobic and facultative anaerobic organisms.
In general, recommended single agents for intra-abdominal infection include second-generation cephalosporins with anaerobic coverage, beta-lactam/beta-lactamase inhibitor agents and carbapenems. Recommended combination regimens include cefuroxime or a third- or fourth-generation cephalosporin plus an antianaerobic agent, or aztreonam plus clindamycin, or ciprofloxacin plus metronidazole or an aminoglycoside plus an antianaerobe (6).
For higher risk patients, single agent antibiotic regimens are limited to imipenem/cilastatin, meropenem or piperacillin/tazobactam. Acceptable combination regimens for the higher risk patient include aminoglycosides plus an antianaerobe, or aztreonam plus clindamycin, or ciprofloxacin plus metronidazole or a third- or fourth-generation cephalosporin plus an antianaerobe (6).
Table 1-Surgical Infection Society Intra-Abdominal
Infection Antimicrobial Therapy Guidelines
|
# of Antibx
|
All patients
|
High-risk patients
|
|
Single Agent Therapy
|
- Second generation cephalosporin with anaerobic activity
- Beta-lactam/beta-lactamase inhibitor
- Carbapenem
|
- Carbapenem
- Piperacillin/tazobactam
|
|
Combination Therapy
|
- Aminoglycoside + anti-anaerobe
- Aztreonam+ clindamycin
- Ciprofloxacin+ metronidazole
- Third or fourth generation cephalosporin+ anti-anaerobe
|
- Aminoglycoside +anti-anaerobe
- Aztreonam + clindamycin
- Ciprofloxacin+ metronidazole
- Third or fourth generation cephalosporin + anti-anaerobe
|
Case continuation…
Once in the SICU after surgery, nutritional assessment revealed inadequate protein- intake related to nausea, vomiting and diarrhea in the ten days before hospital admission. Weight at admission: 94.8 kg, BMI: 29, serum albumin: 2.4 mg/dl, serum prealbumin: 8.1 mg/dl. As a consequence of multiple unsuccessful attempts at post-pyloric feeding tube placement, the patient received no nutritional support in the two days after intubation.
Rationale: The nutritional support of critically ill patients is a subject of great interest. Historically, nutritional support in the critical care setting was seen as adjunctive care, focused on providing exogenous fuels to help the patient during the massive catabolic stress state associated with critical illness. Recently, there has been a shift in emphasis toward nutritional therapy in an effort to prevent oxidative cellular injury and even favorably modulate the immune response via nutrition (7).
The 2009 Guidelines for Assessment of Nutritional Support Therapy in the adult, critically ill patient from the Society of Critical Care Medicine and the American Society for Parenteral and Enteral Nutrition (7) recommend assessing individual nutrition requirements for all critically ill medical and surgical patients expected to have an ICU stay of more than 2 days, regardless of their previous nutritional condition.
Although serum albumin and prealbumin are frequently used to evaluate depletion of serum protein stores, none of the traditional nutrition assessment tools (including anthropometry) has been validated for critically ill patients. However, initial clinical evaluation should consider nutrient intake before admission, admission weight, comorbidities and gastrointestinal function before initiating feeding support.
Enteral nutrition (EN) is the preferred route of feeding over parenteral nutrition (PN) and should be started (if not contraindicated), even in the absence of bowel sounds, within the first 24-48 hours following ICU admission.
In the presented case, an important issue to be considered in initiating nutrition in this patient is the hemodynamic compromise. In this situation, the 2009 guidelines suggest withholding EN until the patient is “fully resuscitated and/or stable”. However, this recommendation has a grade of E, the lowest grade used for these guidelines. If EN is not feasible over the first 7 days and the patient was previously healthy before hospitalization, no nutritional support should be provided. However, if the patient has evidence of protein-calorie malnutrition at admission and EN is not feasible or available, PN should be initiated as soon as possible (7).
Case continuation…
On the second day at SICU, the patient was still febrile, tachycardic, oliguric and requiring high doses of norepinephrine to maintain MAP over 70 mm Hg. Chest radiograph was unchanged and PaO2/FiO2 rate was 84. All cultures were still negative at this point.
Rationale: Activated protein C has been given safely to surgical patients with severe sepsis. Patients with severe sepsis and a higher baseline likelihood of death may benefit from the administration of activated protein C (8, 9).
Activated protein C is administered at a dose of 24 mcg/kg of body weight/hr at a constant rate for a total duration of 96 hours. The activated protein C infusion may be interrupted 1-2 hours before and resumed 1 hour after any simple percutaneous procedure. Activated protein C may not be resumed until 12 hours after major operative procedures as long as bleeding complications were absent (8).
Administration of activated protein C does not improve mortality in less sick patients, such as those with single organ failure or APACHE II score less than 25 (10).
Case continuation…
Vasopressor requirements persisted on his third SICU day and a pulmonary artery catheter was placed. Hemodynamic measurements revealed adequate filling pressures, but low cardiac index consistent with previously described systolic dysfunction. The norepinephrine was weaned to off and dobutamine was started to augment contractility. His multi-organ failure improved and the inotropic support was weaned off after 48 hours. The following day, he was extubated and had continued resolution of his renal failure with increasing urine output. He was transferred out to the floor on SICU day 7.